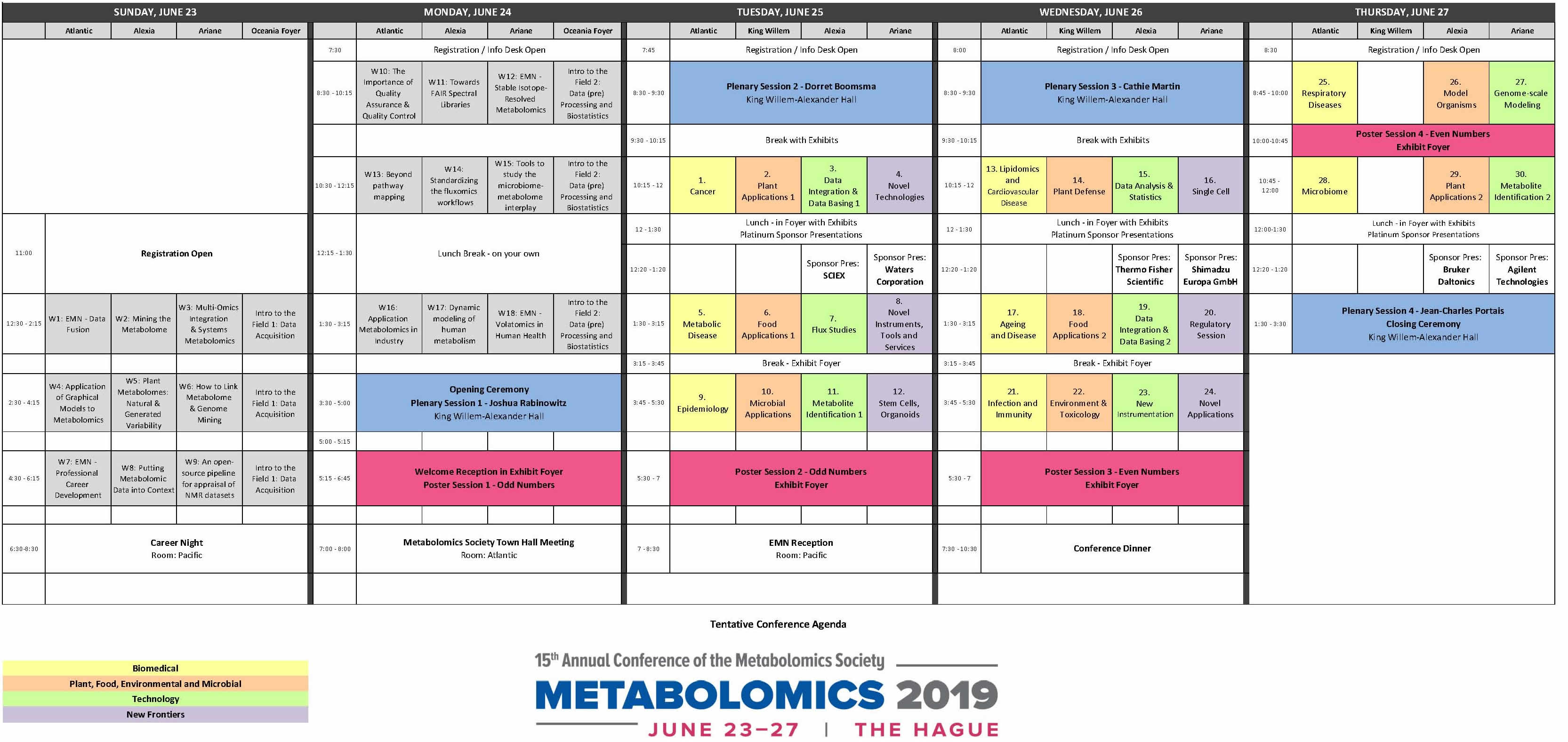
Review the full conference agenda below:
Please click on the image to see it full size.
Click here to download a PDF version of the schedule.
Detailed Program – PDFs
- Schedule PDF – outline of all oral sessions
- Complete Abstract Book – outline of all oral and poster abstracts
Note: a printed program book will not be available at the conference, please download the documents above and utilize the mobile app to stay current with all sessions! Details about accessing the mobile app were e-mailed to all attendees on June 19.
Agenda Highlights
Check out these NEW sessions and features at the 2019 conference:
BioInformatics Hub
During the Breaks and Lunches, click here for more info.
Speaker Meet & Greet
During the Breaks and Lunches
Check-in with Session Keynotes and Plenary Keynotes (as available) at the designated Meet & Greet tables during the next break following their talk.
Session 8 – Vendor Session: Novel Instrumentations, Tools and Services (presented by Platinum and Gold sponsors)
Tuesday, June 25, 1:30 pm – 3:15 pm
By participating in this session you will get an overview of the latest developments in instrumentation and tools (from our 6 platinum sponsors) and services (from our 4 gold sponsors). Each company will highlight their latest developments in a 7-minute flash presentation. The session is scheduled at the start of the conference, allowing you to determine which exhibitors will provide the most valuable follow-up visit at their booth during the event. The interactive session also has 2 panel discussions, moderated by Thomas Hankemeier and David Wishart.
Session 20 – Regulatory Session: Translating metabolomics from academic science into regulatory practice: challenges and progress in pharmacology and toxicology
Wednesday, June 26, 1:30 pm – 3:15 pm
While metabolomics has become a mature and widely used technology in academic research, its application to regulatory science has been limited to date. Several factors contribute to this slow uptake, the most commonly cited roadblocks are the lack of standardisation, validation and reporting formats for metabolomics. Recently, progress has been made in translating ‘omics technologies (including metabolomics) from academic science towards regulatory practice in the fields of pharmacology and toxicology, specifically for drug and chemical safety, respectively. This timely session at Metabolomics-2019 will introduce and review some of the opportunities, challenges and recent progress in this translational activity. While focused on drug and chemical safety legislation in Europe, the progress reported will have much wider implications for metabolomics in international regulatory practice. Talks will be presented by regulators, industry and academic scientists to provide a balanced perspective, and include a panel discussion chaired by Dr. Pim Leonards (Vrije Universiteit Amsterdam).
